Beginnings of Cellular Life
Morowitz proposes that one of the earliest steps on the path to life is the formation of a protocell, a "self-organized, endogenously ordered, spherical collection of lipids" (Wikipedia).
In the H2O molecule the oxygen atom pulls the hydrogen's electrons closer to its nucleus, causing the molecule to have an uneven charge distribution:
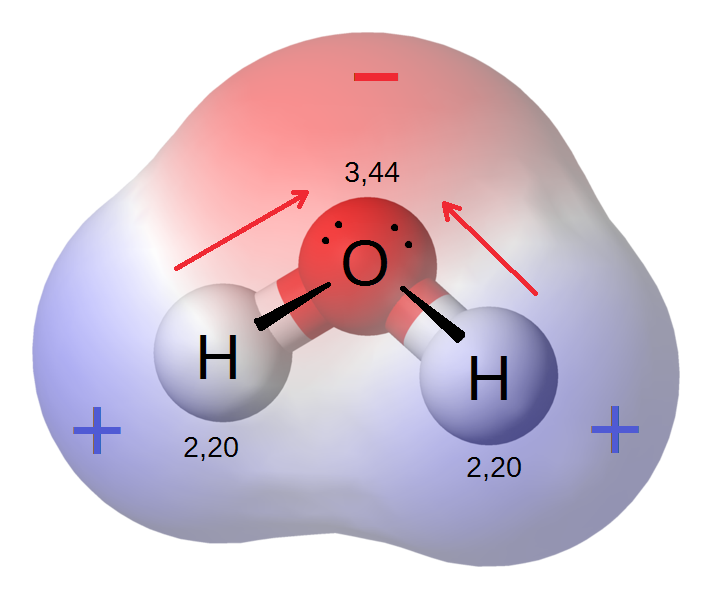
Due to this uneven charge distribution, H2O is a polar molecule. Since unlike charges attract, liquid water consists of many water molecules constantly pulling on each other.
Non-polar molecules have an even charge distribution, and are neither attracted nor repulsed from the polar water molecule. Non-polar substances, like oil, in water naturally aggregate.
Molecules are not necessarily just polar or non-polar. Amphiphiles have a polar head and a non-polar tail:
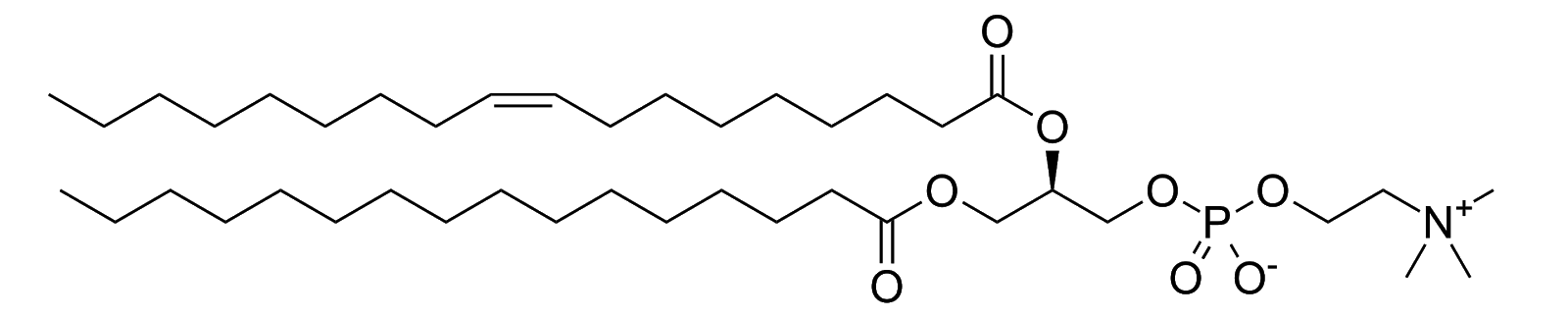
Non-polar tail on left, polar head on right
Like oil, the non-polar tails of amphiphiles naturally aggregate in water. However, due to the polar head, they can’t just lump together randomly. Instead they form structures, including vesicles made of bilipid membranes:
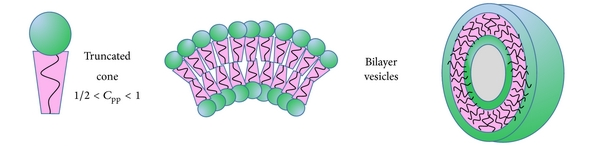
In order: cartooned amphiphile, membrane cross-section, vesicle
The formation of this vesicle "represents a local Gibbs free-energy minimum and can form spontaneously under the appropriate conditions." (pg 94):
Morowitz refers to the bilipid membrane and inner aqueous solutions as phases in the thermodynamic sense: "a region of space throughout which all physical properties of a material are essentially uniform" (Wikipedia).
Linking to Friston, the vesicle can be seen as a Markov blanket. The membrane mediates the way the state space of the inner space can evolve as "the hydrophobic portion of the bilayer has a very low solubility for polar compounds, the inner and outer [ocean] phases can maintain different chemical compositions." (pg 96)